Here are the essential concepts you must grasp in order to answer the question correctly.
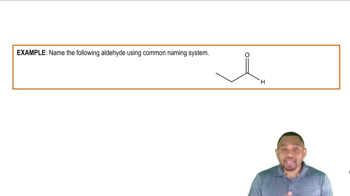
Aldehyde Structure of Glucose
Glucose is a simple sugar that can exist in an open-chain form as an aldehyde. In this form, it has a carbonyl group (C=O) at the first carbon atom, which is crucial for understanding its reactivity and ability to form cyclic structures. The aldehyde group plays a key role in the formation of hemiacetals when glucose reacts with its hydroxyl groups.
Recommended video:
Naming Aldehydes Example 2
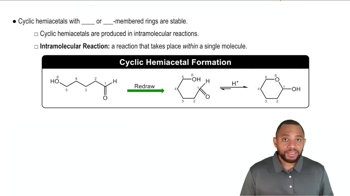
Cyclic Hemiacetal Formation
Cyclic hemiacetals are formed when a hydroxyl group reacts with the carbonyl group of an aldehyde, resulting in a ring structure. In glucose, this process can occur at different carbon atoms, leading to different cyclic forms. The position of the hydroxyl group that reacts determines whether the ring closes at C3 or C4, affecting the resulting anomeric carbon and the overall structure of the sugar.
Recommended video:
Cyclic Hemiacetals Concept 2
Anomeric Carbon
The anomeric carbon is the carbon atom in a sugar that was originally part of the carbonyl group and becomes a new chiral center upon cyclization. In glucose, the anomeric carbon is C1, and its configuration (alpha or beta) influences the properties and reactivity of the sugar. Understanding the concept of the anomeric carbon is essential for predicting the behavior of glucose in biochemical processes.
Recommended video:
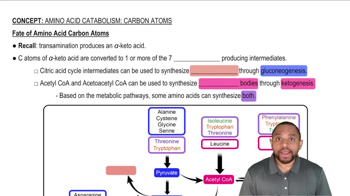
Amino Acid Catabolism: Carbon Atoms Concept 2
 Verified step by step guidance
Verified step by step guidance