Here are the essential concepts you must grasp in order to answer the question correctly.
Lanthanides
The elements from cerium (Ce) to lutetium (Lu) are known as lanthanides, a series of 15 metallic elements in the periodic table. They are characterized by their similar properties, including high melting points and reactivity, and are often used in various high-tech applications such as catalysts and magnets.
Recommended video:
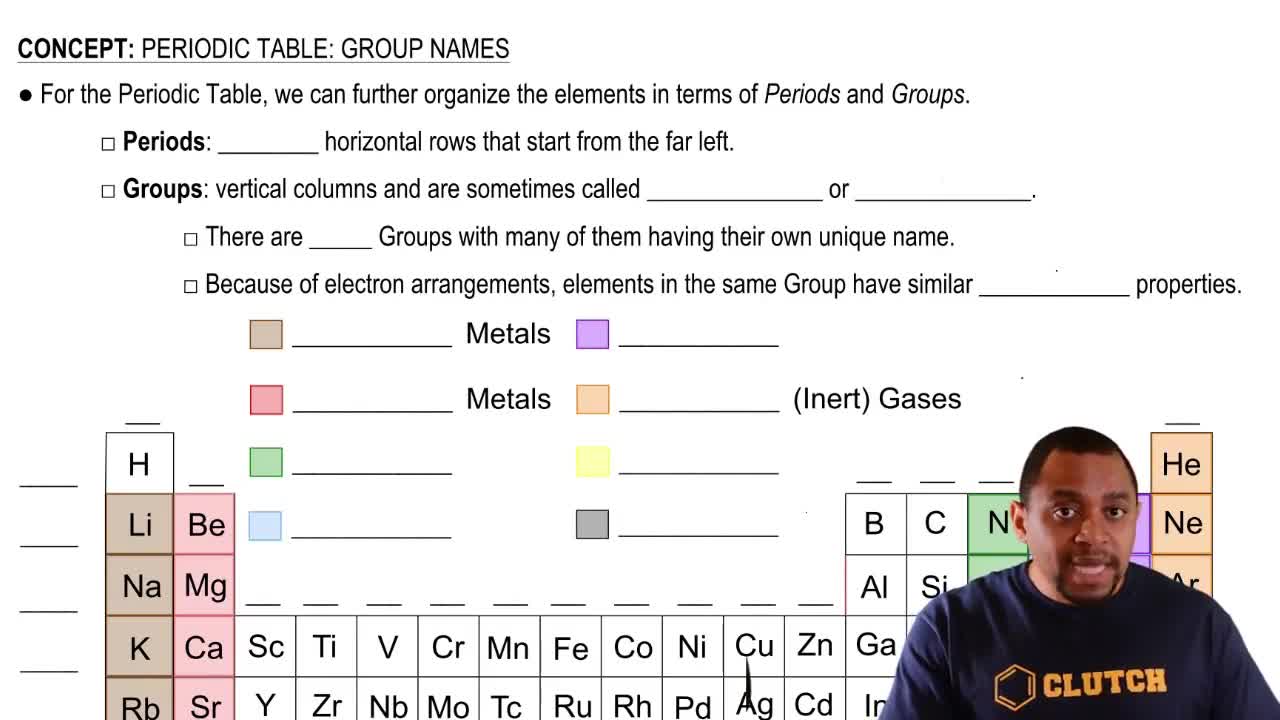
Periodic Table: Group Names
Metals vs. Nonmetals
Metals are typically characterized by their ability to conduct electricity and heat, malleability, ductility, and luster. In contrast, nonmetals are generally poor conductors and are more brittle in solid form. The lanthanides are classified as metals due to their metallic properties and behavior in chemical reactions.
Recommended video:
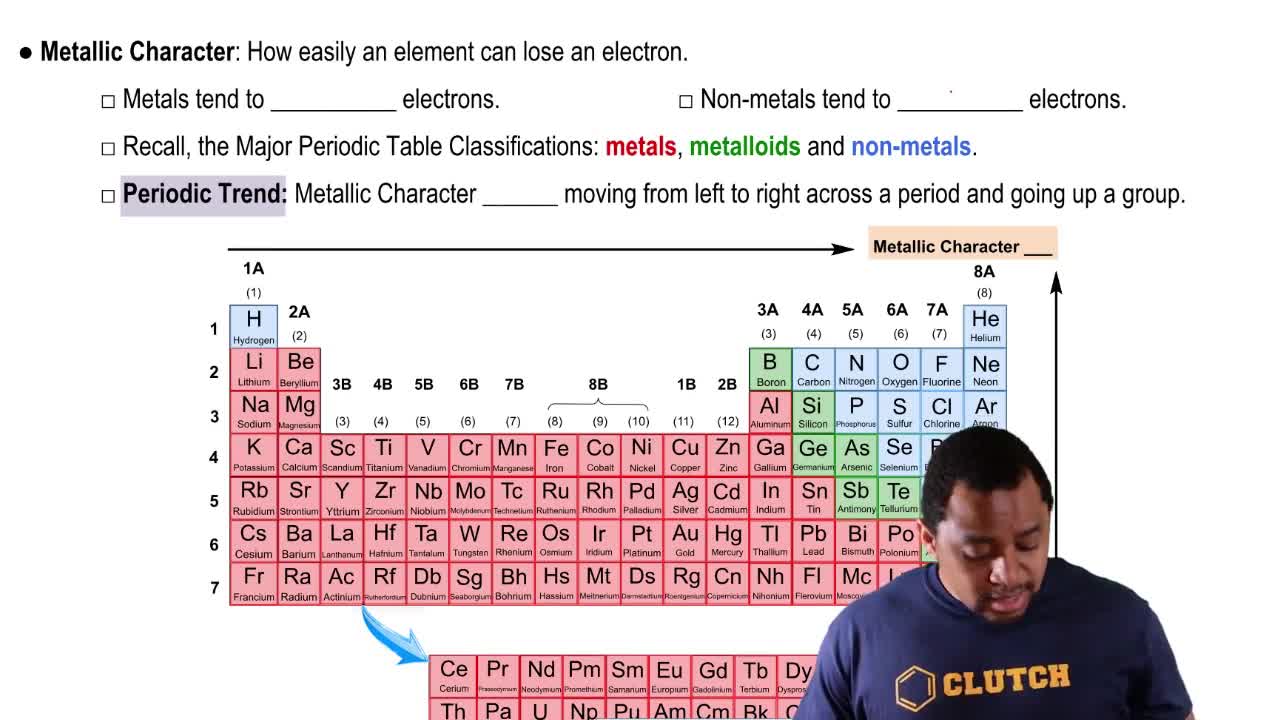
Periodic Trend: Metallic Character
Electron Configuration
The subshell being filled by electrons in lanthanides is the 4f subshell. As you move from cerium to lutetium, electrons are progressively added to this subshell, which is responsible for the unique chemical properties of these elements. Understanding electron configuration is crucial for predicting the behavior of elements in chemical reactions.
Recommended video:
The Electron Configuration: Condensed
 Verified step by step guidance
Verified step by step guidance