Classify each of the following monosaccharides by the type of carbonyl group and the number of carbons (for example, a monosaccharide with an aldehyde and three carbons is an aldotriose).
(a) <IMAGE>
 Verified step by step guidance
Verified step by step guidance

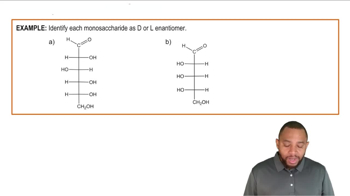
Classify each of the following monosaccharides by the type of carbonyl group and the number of carbons (for example, a monosaccharide with an aldehyde and three carbons is an aldotriose).
(a) <IMAGE>
Draw the Fischer projection for the enantiomer (mirror image) of each of the following:
(a) <IMAGE>
d-Altrose
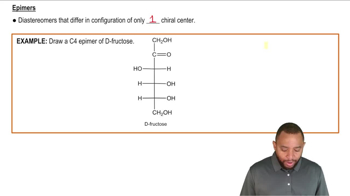
Classify structures A, B, and C in the figure as being either an enantiomer or a diastereomer of d-galactose.
<IMAGE>
Identify the monosaccharide that fits each of the following descriptions:
(a) also referred to as dextrose
Identify the monosaccharide that fits each of the following descriptions:
(a) in combination with glucose produces the disaccharide lactose
ALLIED Health The sugar alcohol ribitol is a component of the vitamin riboflavin and the energy transfer molecule FAD. Ribitol is formed when the monosaccharide ribose undergoes reduction at carbon 1. Draw the structure of ribitol.
<IMAGE>
d-Ribose