Mark the chiral centers in the following molecules, if any, with an asterisk (*):
(a) <IMAGE>
 Verified step by step guidance
Verified step by step guidance


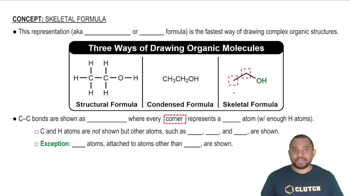
Mark the chiral centers in the following molecules, if any, with an asterisk (*):
(a) <IMAGE>
Mark the chiral centers in the following molecules, if any, with an asterisk (*):
(d) <IMAGE>
Ritalin®
ALLIED Health Mark the chiral centers in the following molecules, if any, with an asterisk (*):
(b) <IMAGE>
Pantothenic Acid, a B vitamin
Alkanes are also referred to as saturated hydrocarbons. Explain the meaning of the term hydrocarbon. Why are alkanes called saturated hydrocarbons?
Give the skeletal structure and name of the straight-chain alkanes whose molecular formula is shown.
(a) C₃H₈
Give the skeletal structure and name of the straight-chain alkanes whose molecular formula is shown.
(b) C₁₀H₂₂