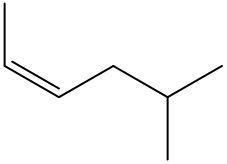
Now before we get into naming alkenes, recall that alkenes possess a carbon-carbon double bond. And the set of rules for naming alkenes is unique. Here we're going to say that the modification is, we're going to modify the ending from -ane to -ene. So we're thinking of an alkane, but because there's a presence of a double bond, it becomes an alkene. This naming convention will include terms like cis and trans, which we'll go into. We still have to give the location of our substituents. We have to give the location of our parent carbon chain in terms of the double bond, and then we have our modifier where we change the ending from -ane to -ene. Now here recall that geometric isomers have a different spatial orientation around a double bond. And remember that rotation is not possible around a double bond, which is a pi bond. Now, we need to indicate which side of the double bond both groups are on, where each group lies. And we do this by saying cis or trans. This only applies when there are two groups around a pi bond. So if we take a look here, we have our two double-bonded carbons here, and we have our two groups, which happen to be these methyl groups. We create a border; this border dissects right through the double-bonded carbons. And you're either here on the bottom side, or you're here on the top side, so you're on different sides of the pi bond. Both methyl groups happen to be on the same side. When they're on the same side, we say that this is cis.
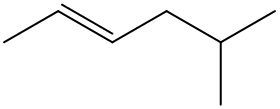
Now, here if we take a look at this alkene, this is a geometric isomer with the same molecular formula, same connections, just a slight difference in spatial orientation. Again, here are our two double-bonded carbons. We create a border by cutting straight through them. Now we have one methyl group up here, and one methyl group down here. They're on different sides of this border that we've created. So if they're on different sides, that means that they are trans. So here we have a cisalkene on the left, and a transalkene on the right.
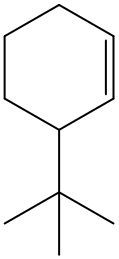
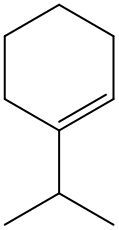
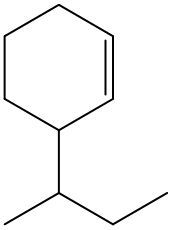
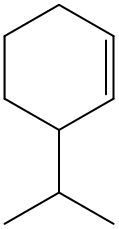
Now, if we're dealing with a cyclic alkene, we're going to say there are no cis or trans within the ring. So we don't have to worry about this type of notation. So again, for us to be cis or trans, we need to have two groups that are connected to our double-bonded carbons. If they're on the same side it's cis, if they're on opposite sides it's trans.