Textbook Question
How do covalent bonds differ from hydrogen bonds? Define base complementarity.
772
views

 Verified step by step guidance
Verified step by step guidance


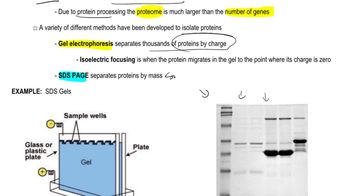
How do covalent bonds differ from hydrogen bonds? Define base complementarity.
List three main differences between DNA and RNA.
What are the three major types of RNA molecules? How is each related to the concept of information flow?
What is the physical state of DNA after it is heated and denatured?
What is the hyperchromic effect? How is it measured? What does Tₘ imply?
Why is Tₘ related to base composition?