A chemist wants to make 3.00 L of a 0.250 M NaNO3 solution. What mass of NaNO3 (in g) should the chemist use?
To what volume should you dilute 50.0 mL of a 12 M stock HNO3 solution to obtain a 0.100 M HNO3 solution?

Verified Solution
Key Concepts
Dilution Principle
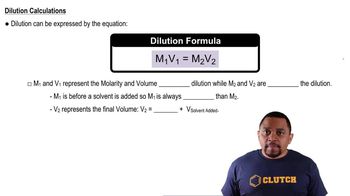
Molarity (M)

Volume Calculation

If 255 mL of a 2.25 M sucrose solution is diluted to 800.0 rnL, what is the molarity of the diluted solution?
If 2.50 L of a 4.80 M MgBr2 solution is diluted to 35.0 L, what is the molarity of the diluted solution?
What is the minimum amount of 6.0 M H2SO4 necessary to produce 25.0 g of H2(g) according to the reaction between aluminum and sulfuric acid? 2 Al(s) + 3 H2SO4(aq) → Al2(SO4)3(aq) + 3 H2(g)
Consider the reaction: Li2S(aq) + Co(NO3)2(aq) → 2 LiNO3(aq) + CoS(s) What volume of 0.150 M Li2S solution is required to completely react with 225 mL of 0.175 M Co(NO3)2?
What is the molarity of ZnCl2 that forms when 25.0 g of zinc completely reacts with CuCl2 according to the following reaction? Assume a final volume of 275 mL. Zn(s) + CuCl2(aq) → ZnCl2(aq) + Cu(s)
