A laboratory procedure calls for making 100.0 mL of a 1.30 M K2SO4 solution. What mass of K2SO4 (in g) is needed?
Ch.5 - Introduction to Solutions and Aqueous Solutions
Chapter 5, Problem 37
If 2.50 L of a 4.80 M MgBr2 solution is diluted to 35.0 L, what is the molarity of the diluted solution?
 Verified step by step guidance
Verified step by step guidance1
Identify the initial conditions: initial volume (V1) = 2.50 L and initial molarity (M1) = 4.80 M.
Identify the final volume after dilution: final volume (V2) = 35.0 L.
Use the dilution formula: M1 * V1 = M2 * V2, where M2 is the molarity of the diluted solution.
Rearrange the formula to solve for M2: M2 = (M1 * V1) / V2.
Substitute the known values into the equation to find M2.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molarity
Molarity (M) is a measure of concentration defined as the number of moles of solute per liter of solution. It is expressed in moles per liter (mol/L) and is crucial for understanding how much solute is present in a given volume of solution. In this question, the initial molarity of the MgBr2 solution is given, which is essential for calculating the final concentration after dilution.
Recommended video:
Guided course

Molarity
Dilution
Dilution is the process of reducing the concentration of a solute in a solution, typically by adding more solvent. The dilution equation, M1V1 = M2V2, relates the initial and final molarities (M1 and M2) and volumes (V1 and V2) of the solution. This concept is key to solving the question, as it allows us to determine the new molarity after the solution is diluted from 2.50 L to 35.0 L.
Recommended video:
Guided course
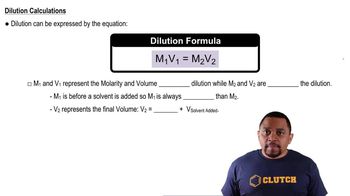
Dilution Equation
Conservation of Moles
The conservation of moles principle states that the number of moles of solute remains constant before and after dilution, assuming no solute is added or removed. This principle is fundamental in dilution calculations, as it allows us to equate the product of initial molarity and volume to the product of final molarity and volume. Understanding this concept is essential for accurately determining the molarity of the diluted solution.
Recommended video:
Guided course
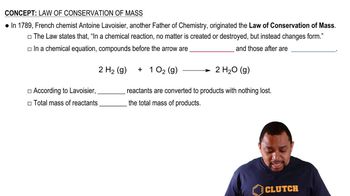
Law of Conservation of Mass
Related Practice
Textbook Question
Textbook Question
A chemist wants to make 3.00 L of a 0.250 M NaNO3 solution. What mass of NaNO3 (in g) should the chemist use?
1
rank
Textbook Question
If 255 mL of a 2.25 M sucrose solution is diluted to 800.0 rnL, what is the molarity of the diluted solution?
Textbook Question
To what volume should you dilute 50.0 mL of a 12 M stock HNO3 solution to obtain a 0.100 M HNO3 solution?
1716
views
Textbook Question
What is the minimum amount of 6.0 M H2SO4 necessary to produce 25.0 g of H2(g) according to the reaction between aluminum and sulfuric acid? 2 Al(s) + 3 H2SO4(aq) → Al2(SO4)3(aq) + 3 H2(g)
5198
views
4
rank
Textbook Question
Consider the reaction: Li2S(aq) + Co(NO3)2(aq) → 2 LiNO3(aq) + CoS(s) What volume of 0.150 M Li2S solution is required to completely react with 225 mL of 0.175 M Co(NO3)2?
1
views
