Here are the essential concepts you must grasp in order to answer the question correctly.
Hydrogenation
Hydrogenation is a chemical reaction that involves the addition of hydrogen (H2) to an unsaturated compound, typically an alkene or alkyne, converting it into a saturated compound. This process is commonly used in organic chemistry to reduce double or triple bonds, resulting in alkanes. Catalysts, such as palladium, platinum, or nickel, are often employed to facilitate the reaction and increase its efficiency.
Recommended video:
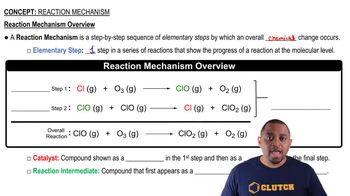
Reaction Mechanism
The reaction mechanism describes the step-by-step sequence of elementary reactions by which a chemical change occurs. Understanding the mechanism of hydrogenation is crucial, as it involves the formation of a transition state and the breaking and forming of bonds. This knowledge helps predict the products of the reaction and the conditions required for it to proceed effectively.
Recommended video:
Reaction Mechanism Overview
Saturation and Unsaturation
In organic chemistry, saturation refers to the presence of single bonds between carbon atoms in a molecule, while unsaturation indicates the presence of double or triple bonds. Saturated compounds, such as alkanes, are generally more stable and less reactive than unsaturated compounds, like alkenes and alkynes. Recognizing the degree of saturation is essential for predicting the outcomes of hydrogenation reactions.
Recommended video:
Saturated and Unsaturated Hydrocarbons