Consider the gas-phase reaction: H2(g) + I2(g) → 2 HI(g) The reaction was experimentally determined to be first order in H2 and first order in I2. Consider the proposed mechanisms. Proposed mechanism I: H2(g) + I2(g) → 2 HI(g) Single step Proposed mechanism II: I2(g) Δk1k-12 I(g) Fast H2( g) + 2 I( g) → k22 HI( g) Slow a. Show that both of the proposed mechanisms are valid.
A certain substance X decomposes. Fifty percent of X remains after 100 minutes. How much X remains after 200 minutes if the reaction order with respect to X is (c) second order?

Verified Solution
Key Concepts
Reaction Order

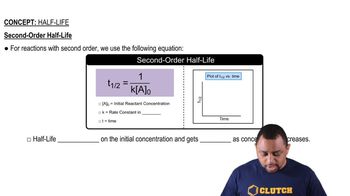
Half-Life in Second-Order Reactions
Integrated Rate Law for Second-Order Reactions

Consider the gas-phase reaction: H2(g) + I2(g) → 2 HI(g) The reaction was experimentally determined to be first order in H2 and first order in I2. Consider the proposed mechanisms. Proposed mechanism I: H2(g) + I2(g) → 2 HI(g) Single step Proposed mechanism II: I2(g) Δk1k-12 I(g) Fast H2( g) + 2 I( g) → k22 HI( g) Slow b. What kind of experimental evidence might lead you to favor mechanism II over mechanism I?
Phosgene (Cl2CO), a poison gas used in World War I, is formed
by the reaction of Cl2 and CO. The proposed mechanism for the
reaction is:
Cl2Δ2 Cl (fast, equilibrium)
Cl + COΔClCO (fast, equilibrium)
ClCO + Cl2¡Cl2CO + Cl (slow)
The half-life for radioactive decay (a first-order process) of plutonium- 239 is 24,000 years. How many years does it take for one mole of this radioactive material to decay until just one atom remains?
Ethyl chloride vapor decomposes by the first-order reaction: C2H5Cl → C2H4 + HCl The activation energy is 249 kJ/mol, and the frequency factor is 1.6⨉1014 s-1. Find the value of the rate constant at 710 K.
Ethyl chloride vapor decomposes by the first-order reaction: C2H5Cl → C2H4 + HCl The activation energy is 249 kJ/mol, and the frequency factor is 1.6⨉1014 s-1. What fraction of the ethyl chloride decomposes in 15 minutes at this temperature?
