An automobile tire has a maximum rating of 38.0 psi (gauge pressure). The tire is inflated (while cold) to a volume of 11.8 L and a gauge pressure of 36.0 psi at a temperature of 12.0 °C. On a hot day, the tire warms to 65.0 °C, and its volume expands to 12.2 L. Does the pressure in the tire exceed its maximum rating? (Note: The gauge pressure is the difference between the total pressure and atmospheric pressure. In this case, assume that atmospheric pressure is 14.7 psi.)
A 1.0-L container of liquid nitrogen is kept in a closet measuring 1.0 m by 1.0 m by 2.0 m. Assuming that the container is completely full, that the temperature is 25.0 °C, and that the atmospheric pressure is 1.0 atm, calculate the percent (by volume) of air that is displaced if all of the liquid nitrogen evaporates. (Liquid nitrogen has a density of 0.807 g/mL.)

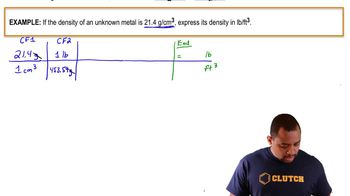
Verified Solution
Key Concepts
Ideal Gas Law

Density and Volume Conversion
Displacement of Air

A weather balloon is inflated to a volume of 28.5 L at a pressure of 748 mmHg and a temperature of 28.0 °C. The balloon rises in the atmosphere to an altitude of approximately 25,000 ft, where the pressure is 385 mmHg and the temperature is -15.0 °C. Assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.
A piece of dry ice (solid carbon dioxide) with a mass of 28.8 g sublimes (converts from solid to gas) into a large balloon. Assuming that all of the carbon dioxide ends up in the balloon, what is the volume of the balloon at 22 °C and a pressure of 742 mmHg?
Which gas sample has the greatest pressure? Assume that all the samples are at the same temperature. Explain.
This picture represents a sample of gas at a pressure of 1 atm, a volume of 1 L, and a temperature of 25 °C. Draw a similar picture showing what would happen to the sample if the volume were reduced to 0.5 L and the temperature were increased to 250 °C. What would happen to the pressure?
Aerosol cans carry clear warnings against incineration because of the high pressures that can develop upon heating. Suppose that a can contains a residual amount of gas at a pressure of 755 mmHg and a temperature of 25 °C. What would the pressure be if the can were heated to 1155 °C?
