What volume is occupied by 12.5 g of argon gas at a pressure of 1.05 atm and a temperature of 322 K? Would the volume be different if the sample were 12.5 g of helium (under identical conditions)?
An automobile tire has a maximum rating of 38.0 psi (gauge pressure). The tire is inflated (while cold) to a volume of 11.8 L and a gauge pressure of 36.0 psi at a temperature of 12.0 °C. On a hot day, the tire warms to 65.0 °C, and its volume expands to 12.2 L. Does the pressure in the tire exceed its maximum rating? (Note: The gauge pressure is the difference between the total pressure and atmospheric pressure. In this case, assume that atmospheric pressure is 14.7 psi.)

Verified Solution
Key Concepts
Gauge Pressure vs. Absolute Pressure

Ideal Gas Law

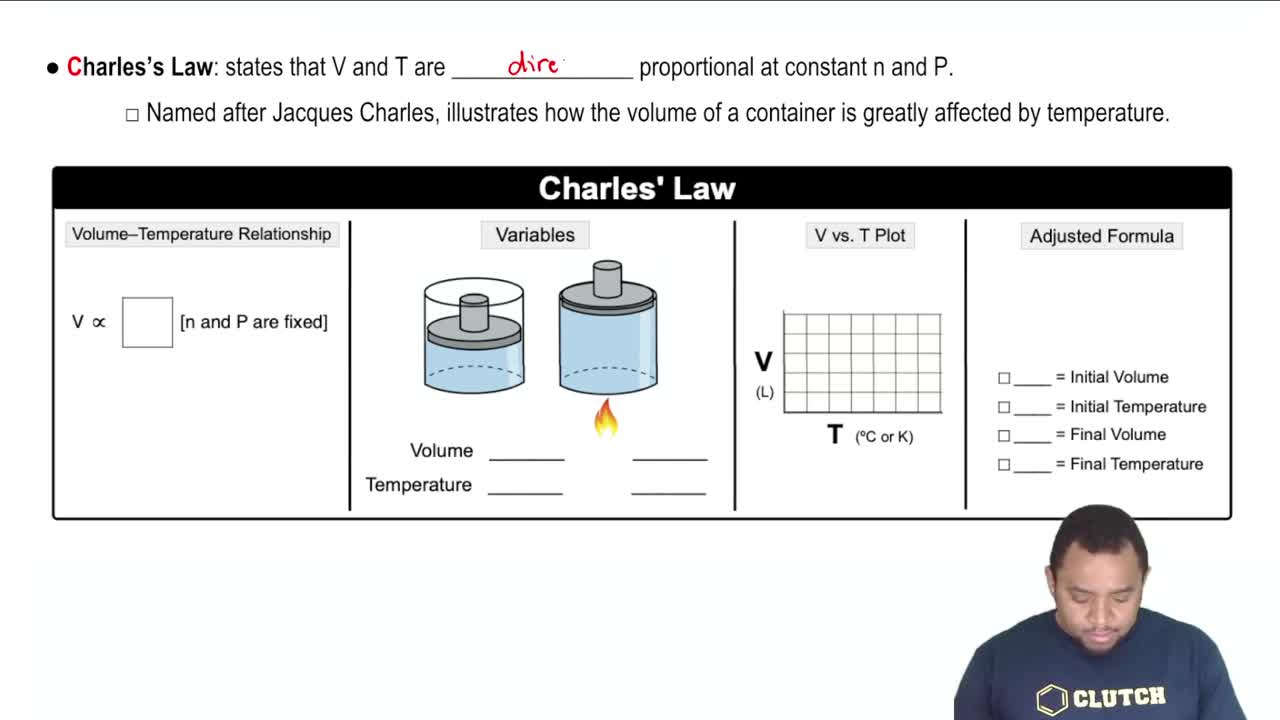
Charles's Law
What is the pressure in a 15.0-L cylinder filled with 32.7 g of oxygen gas at a temperature of 302 K?
What is the temperature of 0.52 mol of gas at a pressure of 1.3 atm and a volume of 11.8 L?
A weather balloon is inflated to a volume of 28.5 L at a pressure of 748 mmHg and a temperature of 28.0 °C. The balloon rises in the atmosphere to an altitude of approximately 25,000 ft, where the pressure is 385 mmHg and the temperature is -15.0 °C. Assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.
A piece of dry ice (solid carbon dioxide) with a mass of 28.8 g sublimes (converts from solid to gas) into a large balloon. Assuming that all of the carbon dioxide ends up in the balloon, what is the volume of the balloon at 22 °C and a pressure of 742 mmHg?
A 1.0-L container of liquid nitrogen is kept in a closet measuring 1.0 m by 1.0 m by 2.0 m. Assuming that the container is completely full, that the temperature is 25.0 °C, and that the atmospheric pressure is 1.0 atm, calculate the percent (by volume) of air that is displaced if all of the liquid nitrogen evaporates. (Liquid nitrogen has a density of 0.807 g/mL.)
