Draw the correct structure for each compound. a. 2-hexene c. 4,4-dimethyl-2-hexene d. 3-ethyl-4-methyl-2-pentene
Ch.22 - Organic Chemistry
Chapter 22, Problem 35
Write structural formulas for each of the nine structural isomers of heptane.
 Verified step by step guidance
Verified step by step guidance1
Identify the molecular formula of heptane, which is C7H16. This indicates that heptane has 7 carbon atoms and 16 hydrogen atoms.
Understand that structural isomers are compounds with the same molecular formula but different structural formulas. This means the connectivity of atoms differs among isomers.
Start by drawing the straight-chain structure of heptane, which is a continuous chain of seven carbon atoms with hydrogen atoms filling the remaining valences.
Create branched isomers by rearranging the carbon skeleton. Begin by moving one carbon from the end of the chain to create a six-carbon chain with a one-carbon branch (methyl group). This is an example of a structural isomer.
Continue creating more isomers by further branching and rearranging the carbon skeleton, ensuring each carbon has four bonds (satisfying the valency of carbon) and the total number of hydrogen atoms remains 16.

Verified Solution
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Structural Isomers
Structural isomers are compounds that have the same molecular formula but differ in the arrangement of atoms. This variation can lead to different physical and chemical properties. In the case of heptane (C7H16), the nine structural isomers arise from different ways to connect the carbon atoms, resulting in distinct structures.
Recommended video:
Guided course

Isomers
Heptane and its Isomers
Heptane is an alkane with seven carbon atoms, and its molecular formula is C7H16. The structural isomers of heptane include straight-chain and branched forms. Understanding the specific arrangements of carbon and hydrogen in these isomers is crucial for drawing their structural formulas accurately.
Recommended video:
Guided course

Isomers
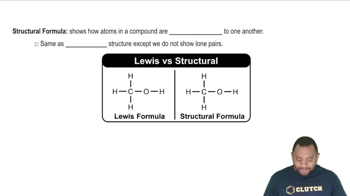
Drawing Structural Formulas
Drawing structural formulas involves representing the arrangement of atoms in a molecule, including bonds between them. For isomers, it is important to depict the connectivity of carbon atoms and the placement of hydrogen atoms. Familiarity with line-angle formulas and the conventions for depicting bonds helps in accurately illustrating the different structural isomers of heptane.
Recommended video:
Guided course
Structural Formula
Related Practice
Textbook Question
670
views
Textbook Question
Based on the molecular formula, determine whether each compound is an alkane, alkene, or alkyne. (Assume that the hydrocarbons are noncyclical and there is no more than one multiple bond.) a. C5H12 b. C3H6 c. C7H12 d. C11H22
637
views
1
rank
Textbook Question
Based on the molecular formula, determine whether each com- pound is an alkane, alkene, or alkyne. (Assume that the hydro- carbons are noncyclical and there is no more than one multiple bond.)
a. C8H16 b. C4H6 c. C7H16 d. C2H2
108
views
Textbook Question
Write structural formulas for any 6 of the 18 structural isomers of octane.
111
views
Textbook Question
Determine whether each compound exhibits optical isomerism c.
285
views
Textbook Question
Determine whether each compound exhibits optical isomerism c.
355
views
