Here are the essential concepts you must grasp in order to answer the question correctly.
Optical Isomerism
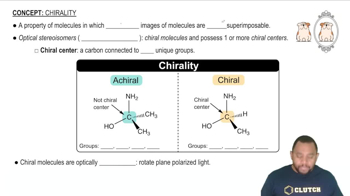
Optical isomerism, also known as chirality, occurs when a molecule can exist in two non-superimposable mirror image forms called enantiomers. These isomers have identical physical properties except for the direction in which they rotate plane-polarized light. The presence of a chiral center, typically a carbon atom bonded to four different substituents, is a key factor in determining whether a compound exhibits optical isomerism.
Recommended video:
Isomerism in Coordination Complexes Example
Chiral Centers
A chiral center, often a carbon atom, is a point in a molecule where four different groups are attached, leading to the possibility of forming two distinct stereoisomers. The arrangement of these groups around the chiral center determines the molecule's chirality. Identifying chiral centers is essential for assessing whether a compound can exhibit optical isomerism.
Recommended video:
Stereoisomerism
Stereoisomerism refers to the phenomenon where molecules have the same molecular formula and connectivity of atoms but differ in the spatial arrangement of their atoms. This category includes optical isomers, which are specifically related to the orientation of substituents around chiral centers. Understanding stereoisomerism is crucial for determining the optical activity of compounds and their potential isomeric forms.
Recommended video: