Textbook Question
Write a nuclear equation for the indicated decay of each nuclide. e. Cr-51 (electron capture)
563
views
 Verified step by step guidance
Verified step by step guidance

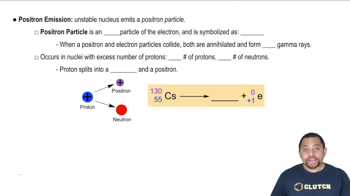

Write a nuclear equation for the indicated decay of each nuclide. e. Cr-51 (electron capture)
Write a nuclear equation for the indicated decay of each nuclide. a. Po-210 (alpha) b. Ac-227 (beta)
Write a nuclear equation for the indicated decay of each nuclide.
c. Tl-207 (beta)
Write a nuclear equation for the indicated decay of each nuclide. e. Pd-103 (electron capture)
Write a partial decay series for Th-232 undergoing the sequential decays: a, b, b, a.
Write a partial decay series for Rn-220 undergoing the sequential decays: a, a, b, b.