Textbook Question
Write a nuclear equation for the indicated decay of each nuclide. a. Po-210 (alpha) b. Ac-227 (beta)
112
views
 Verified step by step guidance
Verified step by step guidance

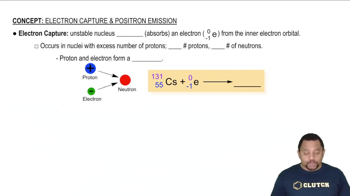

Write a nuclear equation for the indicated decay of each nuclide. a. Po-210 (alpha) b. Ac-227 (beta)
Write a nuclear equation for the indicated decay of each nuclide.
c. Tl-207 (beta)
Write a nuclear equation for the indicated decay of each nuclide.
d. O-15 (positron emission)
Write a partial decay series for Th-232 undergoing the sequential decays: a, b, b, a.
Write a partial decay series for Rn-220 undergoing the sequential decays: a, a, b, b.
Fill in the missing particles in each nuclear equation.
a. ____ → 21785At + 42He
b. 24194Pu → 24195Am + ____
c. 1911Ne → 1910Ne + ____
d. 7534Se + _____ → 7533As