A solution contains a mixture of pentane and hexane at room temperature. The solution has a vapor pressure of 258 torr. Pure pentane and hexane have vapor pressures of 425 torr and 151 torr, respectively, at room temperature. What is the mole fraction composition of the mixture? (Assume ideal behavior.)
Calculate the freezing point and boiling point of each aqueous solution, assuming complete dissociation of the solute. c. 5.5% NaNO3 by mass (in water)
 Verified step by step guidance
Verified step by step guidanceRecommended similar problem, with video answer:

Verified Solution
Key Concepts
Colligative Properties
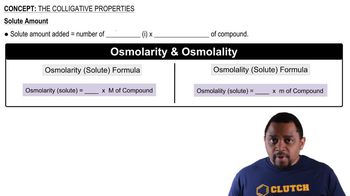
Dissociation of Ionic Compounds

Freezing Point Depression and Boiling Point Elevation Formulas

A glucose solution contains 55.8 g of glucose (C6H12O6) in 455 g of water. Determine the freezing point and boiling point of the solution.
Calculate the freezing point and boiling point of each aqueous solution, assuming complete dissociation of the solute. a. 0.100 m K2S b. 21.5 g of CuCl2 in 4.50⨉102 g water
What mass of salt (NaCl) should you add to 1.00 L of water in an ice cream maker to make a solution that freezes at -10.0 °C? Assume complete dissociation of the NaCl and density of 1.00 g/mL for water.
Use the van't Hoff factors in Table 13.9 to calculate each colligative property: a. the melting point of a 0.100 m iron(III) chloride solution
A 1.2 m aqueous solution of an ionic compound with the formula MX2 has a boiling point of 101.4 °C. Calculate the van't Hoff factor (i) for MX2 at this concentration.
