Textbook Question
Arrange these elements in order of increasing first ionization energy: Si, F, In, N.
1501
views
2
rank
 Verified step by step guidance
Verified step by step guidance

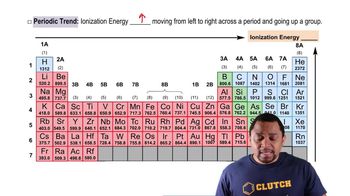
Arrange these elements in order of increasing first ionization energy: Si, F, In, N.
For each element, predict where the 'jump' occurs for successive ionization energies. (For example, does the jump occur between the first and second ionization energies, the second and third, or the third and fourth?) a. Be b. N c. O d. Li
Choose the element with the more negative (more exothermic) electron affinity from each pair. a. Na or Rb
Choose the element with the more negative (more exothermic) electron affinity from each pair. b. B or S
Choose the element with the more negative (more exothermic) electron affinity from each pair. c. C or N d. Li or F