For each element, predict where the 'jump' occurs for successive ionization energies. (For example, does the jump occur between the first and second ionization energies, the second and third, or the third and fourth?) a. Be b. N c. O d. Li
Ch.8 - Periodic Properties of the Elements
Chapter 8, Problem 79b
Choose the element with the more negative (more exothermic) electron affinity from each pair. b. B or S
 Verified step by step guidance
Verified step by step guidance1
Understand the concept of electron affinity: Electron affinity is the energy change that occurs when an electron is added to a neutral atom in the gaseous state to form a negative ion. A more negative electron affinity indicates a greater tendency to accept an electron.
Identify the position of each element in the periodic table: Boron (B) is in Group 13 and Period 2, while Sulfur (S) is in Group 16 and Period 3.
Recall the general trend for electron affinity in the periodic table: Electron affinity generally becomes more negative across a period from left to right and less negative down a group.
Compare the elements based on their positions: Since Sulfur (S) is to the right of Boron (B) in the same period, it is expected to have a more negative electron affinity.
Conclude which element has the more negative electron affinity: Based on periodic trends, Sulfur (S) is expected to have a more negative (more exothermic) electron affinity than Boron (B).

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Affinity
Electron affinity is the amount of energy released when an electron is added to a neutral atom in the gas phase. A more negative electron affinity indicates a greater release of energy, meaning the atom has a stronger attraction for the added electron. This property is crucial for understanding how elements interact with electrons and form ions.
Recommended video:
Guided course

Electron Affinity
Trends in Electron Affinity
Electron affinity varies across the periodic table, generally increasing (becoming more negative) from left to right and decreasing (becoming less negative) from top to bottom. This trend is influenced by atomic size and effective nuclear charge, which affect how tightly an atom can hold onto its electrons. Recognizing these trends helps predict the behavior of elements in chemical reactions.
Recommended video:
Guided course
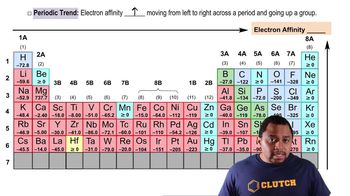
Electron Affinity Trends
Comparison of Elements
When comparing elements like boron (B) and sulfur (S), it is essential to consider their positions in the periodic table. Sulfur, being further to the right and lower in the table than boron, typically has a more negative electron affinity due to its higher effective nuclear charge and smaller atomic radius, which allows it to attract additional electrons more effectively.
Recommended video:
Guided course

Elemental Forms of Elements
Related Practice
Textbook Question
3121
views
Textbook Question
Consider this set of ionization energies. IE1 = 578 kJ/mol IE2 = 1820 kJ/mol IE3 = 2750 kJ/mol IE4 = 11,600 kJ/mol To which third-period element do these ionization values belong?
5849
views
1
rank
1
comments
Textbook Question
Choose the element with the more negative (more exothermic) electron affinity from each pair. a. Na or Rb
2072
views
Textbook Question
Choose the element with the more negative (more exothermic) electron affinity from each pair. c. C or N d. Li or F
323
views
Textbook Question
Choose the element with the more negative (more exothermic) electron affinity in each pair. a. Mg or S b. K or Cs c. Si or P d. Ga or Br
699
views
1
rank
Textbook Question
Choose the more metallic element from each pair. c. Cl or O
740
views
