Write the electron configuration for each ion. d. Mo3+ e. V3+
Choose the element with the more negative (more exothermic) electron affinity from each pair. c. C or N d. Li or F

Verified Solution
Key Concepts
Electron Affinity

Trends in Electron Affinity
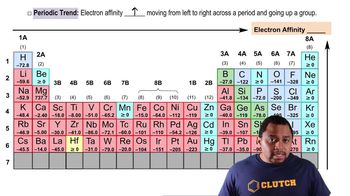
Comparison of Carbon and Nitrogen

Write the full orbital diagram for each element. c. Ne d. He
If core electrons completely shielded valence electrons from nuclear charge (i.e., if each core electron reduced nuclear charge by 1 unit) and if valence electrons did not shield one another from nuclear charge at all, what would be the effective nuclear charge experienced by the valence electrons of each atom? c. O d. C
Use the periodic table to determine each quantity. b. the number of 3d electrons in Cu d. the number of 4d electrons in Zr
Use the periodic table to write an electron configuration for each element. Represent core electrons with the symbol of the previous noble gas in brackets. a. P b. Ge c. I
Determine the number of valence electrons in an atom of each element. a. Ba c. Ni d. S
