Textbook Question
The mass of an evacuated 255 mL flask is 143.187 g. The mass of the flask filled with 267 torr of an unknown gas at 25 °C is 143.289 g. Calculate the molar mass of the unknown gas.
2171
views
2
rank

 Verified step by step guidance
Verified step by step guidance
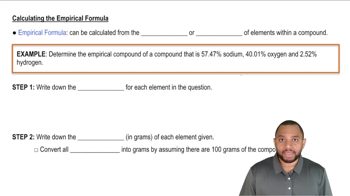


The mass of an evacuated 255 mL flask is 143.187 g. The mass of the flask filled with 267 torr of an unknown gas at 25 °C is 143.289 g. Calculate the molar mass of the unknown gas.
A gaseous hydrogen- and carbon-containing compound is decomposed and found to contain 82.66% carbon and 17.34% hydrogen by mass. The mass of 158 mL of the gas, measured at 556 mmHg and 25 °C, was 0.275 g. What is the molecular formula of the compound?
Consider the reaction: 2 Ag2O(s) → 4 Ag(s) + O2(g) If this reaction produces 15.8 g of Ag(s), what total volume of gas can be collected over water at a temperature of 25 °C and a total pressure of 752 mmHg?