Here are the essential concepts you must grasp in order to answer the question correctly.
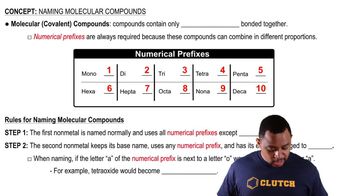
Molecular Compounds
Molecular compounds are formed when two or more nonmetals bond together by sharing electrons. These compounds typically have distinct molecular formulas that indicate the types and numbers of atoms present. Understanding how to write these formulas involves recognizing the elements involved and their respective quantities, often indicated by prefixes in the compound's name.
Recommended video:
Naming Molecular Compounds
Naming Conventions
The naming of molecular compounds follows specific conventions, often using prefixes to denote the number of atoms of each element. For example, 'penta-' indicates five atoms of an element. In the case of phosphorus pentafluoride, the prefix 'penta-' signifies that there are five fluorine atoms bonded to one phosphorus atom, which is crucial for accurately writing the chemical formula.
Recommended video:
Chemical Formula Representation
A chemical formula represents the composition of a compound using element symbols and numerical subscripts. For phosphorus pentafluoride, the formula is PF5, where 'P' stands for phosphorus and 'F' for fluorine, with the subscript '5' indicating that there are five fluorine atoms. This concise representation allows chemists to quickly understand the molecular structure and stoichiometry of the compound.
Recommended video: