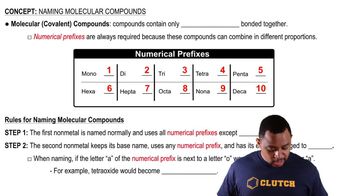
Molecular Compounds
Molecular compounds are formed when two or more nonmetals bond together by sharing electrons. These compounds typically have distinct properties, such as lower melting and boiling points compared to ionic compounds. The naming of molecular compounds often involves using prefixes to indicate the number of atoms of each element present in the molecule.
Recommended video:
Naming Molecular Compounds
Nomenclature of Compounds
The nomenclature of compounds refers to the systematic method of naming chemical substances. For molecular compounds, the names are derived from the elements involved, with prefixes like mono-, di-, tri-, etc., used to denote the number of atoms. For example, in the compound NO, 'N' stands for nitrogen and 'O' for oxygen, and the compound is named nitrogen monoxide.
Recommended video:
Chemical Symbols and Formulas
Chemical symbols and formulas represent the elements and their proportions in a compound. Each element is denoted by a one- or two-letter symbol, with the first letter capitalized. In the case of NO, 'N' represents nitrogen and 'O' represents oxygen, indicating that the compound consists of one nitrogen atom and one oxygen atom.
Recommended video:
 Verified step by step guidance
Verified step by step guidance