Here are the essential concepts you must grasp in order to answer the question correctly.
Isomerism
Isomerism refers to the phenomenon where compounds with the same molecular formula exhibit different structural arrangements. In the case of C5H12, there are multiple structural isomers, meaning that the atoms can be connected in various ways, leading to distinct compounds with different properties.
Recommended video:
Isomerism in Coordination Complexes Example
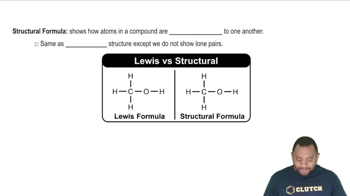
Structural Formula
A structural formula represents the arrangement of atoms within a molecule, showing how the atoms are bonded to each other. It provides insight into the connectivity and spatial orientation of the atoms, which is crucial for understanding the chemical behavior and reactivity of the compound.
Recommended video:
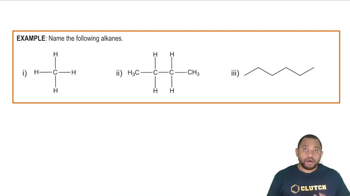
Alkanes
Alkanes are a class of hydrocarbons characterized by single bonds between carbon atoms and follow the general formula CnH2n+2. The molecular formula C5H12 indicates that the compounds in question are alkanes, specifically pentanes, which can exist in different structural forms such as straight-chain and branched isomers.
Recommended video: