Use the mass spectrum of lead to estimate the atomic mass of lead. Estimate the mass and percent intensity values from the graph to three significant figures.
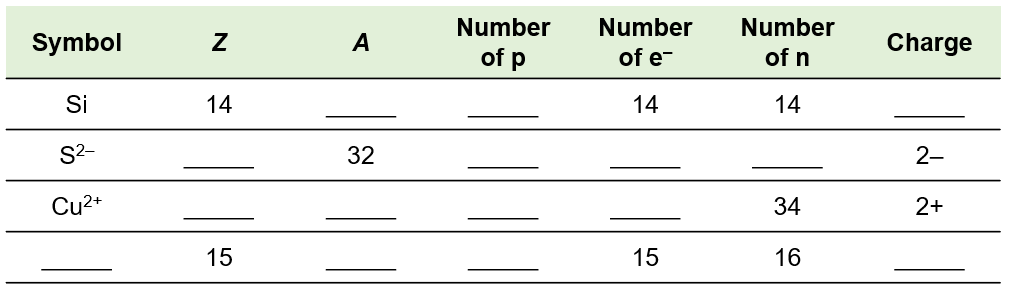
Fill in the blanks to complete the table. Symbol Z A Number of p_x001E_ Number of e_x001D_ Number of n Charge Si 14 ____ ____ 14 14 ____ S2- ____ 32 ____ ____ ____ 2- Cu2+ ____ ____ ____ ____ 34 2+ ____ 15 ____ ____ 15 16 ____
 Verified step by step guidance
Verified step by step guidanceUse the mass spectrum of mercury to estimate the atomic mass of mercury. Estimate the masses and percent intensity values from the graph to three significant figures.
Nuclei with the same number of neutrons but different mass numbers are called isotones. Write the symbols of four isotones of 236Th.
Fill in the blanks to complete the table. Symbol Z A Number of p Number of e− Number of n Charge ______ 8 ______ ______ ______ 8 2- Ca2 + 20 ______ ______ ______ 20 ______ Mg2 + ______ 25 ______ ______ 13 2+ N3 - ______ 14 ______ 10 ______ ______
Neutron stars are composed of solid nuclear matter, primarily neutrons. Assume the radius of a neutron is approximately 1.0×10–13 cm. Calculate the density of a neutron. [Hint: For a sphere V = (4/3)πr3.] Assuming that a neutron star has the same density as a neutron, calculate the mass (in kg) of a small piece of a neutron star the size of a spherical pebble with a radius of 0.10 mm.
Carbon-12 contains six protons and six neutrons. The radius of the nucleus is approximately 2.7 fm (femtometers) and the radius of the atom is approximately 70 pm (picometers). Calculate the volume of the nucleus and the volume of the atom.
