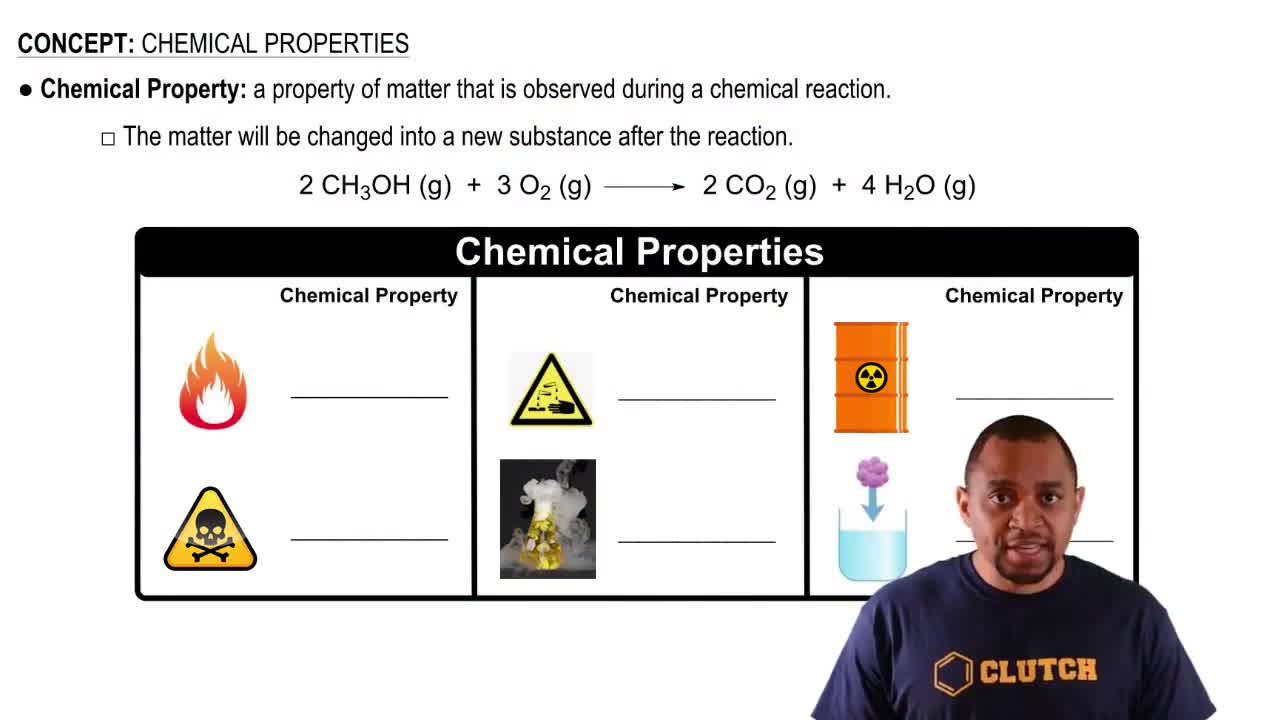
Open Question
Refer to the tabulated values of ∆Gf° in Appendix IIB to calculate E°cell for a fuel cell that employs the reaction between methane gas (CH4) and oxygen to form carbon dioxide and gaseous water.
 Verified step by step guidance
Verified step by step guidance


Refer to the tabulated values of ∆G°f in Appendix IIB to calculate E°cell for the fuel-cell breathalyzer, which employs the following reaction. ((∆G° for HC2H3O2(g) = -374.2 kJ/mol.)
CH3CH2OH(g) + O2(g) → HC2H3O2(g) + H2O(g)
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. a. Zn
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. c. Mn
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. a. Mg
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. b. Cr