Textbook Question
What mass of lead sulfate is formed in a lead–acid storage battery when 1.00 g of Pb undergoes oxidation?
493
views



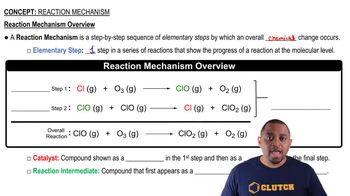
What mass of lead sulfate is formed in a lead–acid storage battery when 1.00 g of Pb undergoes oxidation?
Refer to the tabulated values of ∆G°f in Appendix IIB to calculate E°cell for the fuel-cell breathalyzer, which employs the following reaction. ((∆G° for HC2H3O2(g) = -374.2 kJ/mol.)
CH3CH2OH(g) + O2(g) → HC2H3O2(g) + H2O(g)
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. b. Sn
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. c. Mn
Determine whether or not each metal, if coated onto iron, would prevent the corrosion of iron. a. Mg