Textbook Question
Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base according to the Arrhenius definition. a. HNO3(aq) b. NH4+(aq) d. HC2H3O2(aq)
884
views
 Verified step by step guidance
Verified step by step guidance


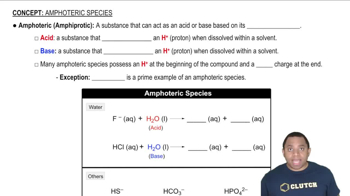
Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base according to the Arrhenius definition. a. HNO3(aq) b. NH4+(aq) d. HC2H3O2(aq)
Identify the Lewis acid and Lewis base from among the reactants in each equation. b. AlBr3 + NH3 ⇌ H3NAlBr3 c. F–(aq) + BF3(aq) ⇌ BF4–(aq)
Calculate [H3O+] and [OH–] for each solution at 25 °C. b. pH = 11.23 c. pH = 2.87
Find the pH of each mixture of acids. b. 0.150 M in HNO2 and 0.085 M in HNO3 c. 0.185 M in HCHO2 and 0.225 M in HC2H3O2 d. 0.050 M in acetic acid and 0.050 M in hydrocyanic acid