Textbook Question
Write the formula for the conjugate base of each acid. d. HF
421
views

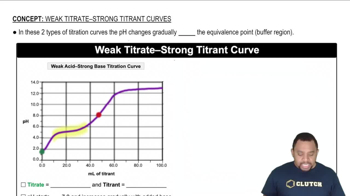


Write the formula for the conjugate base of each acid. d. HF
Write the formula for the conjugate acid of each base. a. NH3 b. ClO4– c. HSO4– d. CO32–
Both H2O and H2PO4– are amphoteric. Write an equation to show how each substance can act as an acid and another equation to show how each can act as a base.
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). b. HCl
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). c. HBr
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). d. H2SO3