Textbook Question
Write the formula for the conjugate base of each acid. c. HCHO2
902
views
 Verified step by step guidance
Verified step by step guidance
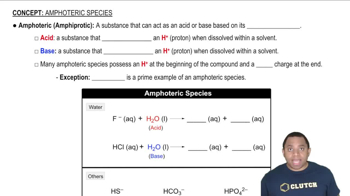


Write the formula for the conjugate base of each acid. c. HCHO2
Write the formula for the conjugate base of each acid. d. HF
Write the formula for the conjugate acid of each base. a. NH3 b. ClO4– c. HSO4– d. CO32–
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). a. HNO3
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). b. HCl
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). c. HBr