Open Question
A system at equilibrium contains I2(g) at a pressure of 0.21 atm and I(g) at a pressure of 0.23 atm. The system is then compressed to half its volume. Find the pressure of each gas when the system returns to equilibrium.
 Verified step by step guidance
Verified step by step guidance


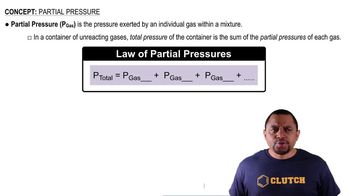
Consider the exothermic reaction: C2H4(g) + Cl2(g) ⇌ C2H4Cl2(g) If you were trying to maximize the amount of C2H4Cl2 produced, which tactic might you try? Assume that the reaction mixture reaches equilibrium. a. increasing the reaction volume b. removing C2H4Cl2 from the reaction mixture as it forms c. lowering the reaction temperature d. adding Cl2
Consider the endothermic reaction: C2H4(g) + I2(g) ⇌ C2H4I2(g) If you were trying to maximize the amount of C2H4I2 produced, which tactic might you try? Assume that the reaction mixture reaches equilibrium. a. decreasing the reaction volume b. removing I2 from the reaction mixture c. raising the reaction temperature d. adding C2H4 to the reaction mixture