Here are the essential concepts you must grasp in order to answer the question correctly.
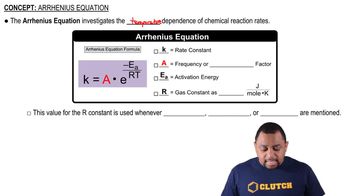
Arrhenius Equation
The Arrhenius equation relates the rate constant of a reaction to temperature and activation energy. It is expressed as k = A * e^(-Ea/RT), where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the universal gas constant, and T is the temperature in Kelvin. This equation shows that as temperature increases, the rate constant typically increases, indicating a higher reaction rate.
Recommended video:
Activation Energy (Ea)
Activation energy is the minimum energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. A higher activation energy means that fewer molecules have sufficient energy to react at a given temperature, resulting in a slower reaction rate. Understanding Ea is crucial for predicting how temperature changes affect reaction rates.
Recommended video:
Temperature Dependence of Reaction Rates
The rate of a chemical reaction is often temperature-dependent, with higher temperatures generally leading to increased reaction rates. This is due to the increased kinetic energy of molecules, which results in more frequent and effective collisions. The relationship between temperature and reaction rate can be quantitatively analyzed using the Arrhenius equation, allowing for the determination of activation energy from rate constants at different temperatures.
Recommended video:
Kw Temperature Dependence