The osmotic pressure of a solution containing 2.10 g of an unknown compound dissolved in 175.0 mL of solution at 25 °C is 1.93 atm. The combustion of 24.02 g of the unknown compound produced 28.16 g CO2 and 8.64 g H2O. What is the molecular formula of the compound (which contains only carbon, hydrogen, and oxygen)?

Verified Solution
Key Concepts
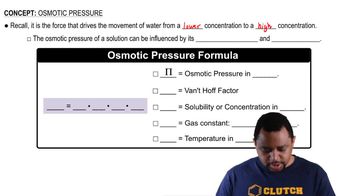
Osmotic Pressure
Combustion Analysis

Empirical and Molecular Formulas

Find the mass of urea (CH4N2O) needed to prepare 50.0 g of a solution in water in which the mole fraction of urea is 0.0770.
A solution contains 10.05 g of unknown compound dissolved in 50.0 mL of water. (Assume a density of 1.00 g/mL for water.) The freezing point of the solution is -3.16 °C. The mass percent composition of the compound is 60.97% C, 11.94% H, and the rest is O. What is the molecular formula of the compound?
A 100.0-mL aqueous sodium chloride solution is 13.5% NaCl by mass and has a density of 1.12 g/mL. What would you add (solute or solvent) and what mass of it to make the boiling point of the solution 104.4 °C? (Use i = 1.8 for NaCl.)
A 50.0-mL solution is initially 1.55% MgCl2 by mass and has a density of 1.05 g/mL. What is the freezing point of the solution after you add an additional 1.35 g MgCl2? (Use i = 2.5 for MgCl2.)
