Find the mass of urea (CH4N2O) needed to prepare 50.0 g of a solution in water in which the mole fraction of urea is 0.0770.
A 100.0-mL aqueous sodium chloride solution is 13.5% NaCl by mass and has a density of 1.12 g/mL. What would you add (solute or solvent) and what mass of it to make the boiling point of the solution 104.4 °C? (Use i = 1.8 for NaCl.)
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Colligative Properties
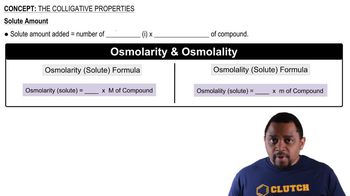
Boiling Point Elevation Formula

Density and Mass Calculations

A solution contains 10.05 g of unknown compound dissolved in 50.0 mL of water. (Assume a density of 1.00 g/mL for water.) The freezing point of the solution is -3.16 °C. The mass percent composition of the compound is 60.97% C, 11.94% H, and the rest is O. What is the molecular formula of the compound?
The osmotic pressure of a solution containing 2.10 g of an unknown compound dissolved in 175.0 mL of solution at 25 °C is 1.93 atm. The combustion of 24.02 g of the unknown compound produced 28.16 g CO2 and 8.64 g H2O. What is the molecular formula of the compound (which contains only carbon, hydrogen, and oxygen)?
A 50.0-mL solution is initially 1.55% MgCl2 by mass and has a density of 1.05 g/mL. What is the freezing point of the solution after you add an additional 1.35 g MgCl2? (Use i = 2.5 for MgCl2.)
