Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
All textbooks McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 153
Problem 153
 McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 153
Problem 153Chapter 8, Problem 153
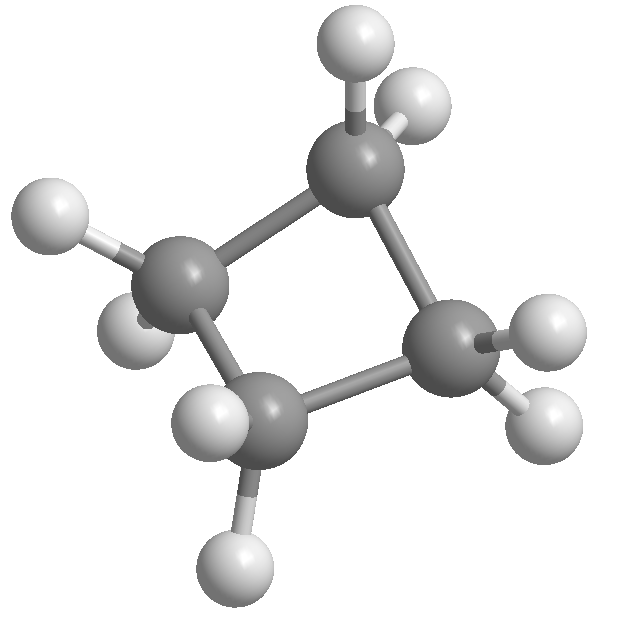
The equilibrium constant Kc for the gas-phase thermal decom-position of cyclopropane to propene is 1.0 * 105 at 500 K:
 Verified Solution
Verified SolutionVideo duration:
2m292
views
Was this helpful?
Video transcript
Related Practice
Textbook Question
Bond vibrations for the symmetric and asymmetric stretch
in methane are illustrated below. Decide whether each vibration
will result in the absorption of IR radiation. Arrows
indicate the movement of atoms during the vibration.
478
views
Textbook Question
Values of Ea = 6.3 kJ>mol and A = 6.0 * 108>1M # s2
have been measured for the bimolecular reaction:
NO1g2 + F21g2S NOF1g2 + F1g2
(b) The product of the reaction is nitrosyl fluoride. Its formula
is usually written as NOF, but its structure is actually
ONF. Is the ONF molecule linear or bent?
361
views
Textbook Question
(b) When xenon absorbs 801 kJ/mol of energy, it is excited into a higher-energy state in which the outermost elec-tron has been promoted to the next available subshell. Write the electron configuration for this excited xenon.
570
views
Textbook Question
Reaction of gaseous fluorine with compound X yields a sin- gle product Y, whose mass percent composition is 61.7% F and 38.3% Cl. (b) Draw an electron-dot structure for Y, and predict the geometry around the central atom.
353
views