Ch.5 - Periodicity & Electronic Structure of Atoms
Chapter 5, Problem 31
What atom has the following orbital-filling diagram?
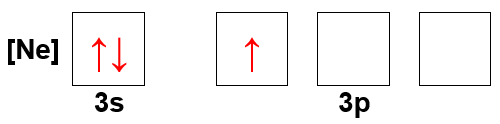
 Verified Solution
Verified SolutionVideo duration:
1mWas this helpful?
Video transcript
Related Practice
Textbook Question
Identify each of the following orbitals, and give n and l quantum numbers for each.
(a)
(b)
660
views
Textbook Question
Where on the blank outline of the periodic table do elements that meet the following descriptions appear? (c) Elements with electrons whose largest principal quantum number is n = 4
681
views
1
rank
Textbook Question
One of the elements shown on the following periodic table has an anomalous ground-state electron configuration. Which is it—red, blue, or green—and why?
444
views
Textbook Question
Which of the following three spheres represents a Ca atom, which an Sr atom, and which a Br atom?
438
views
Textbook Question
Which has the higher frequency, red light or violet light? Which has the longer wavelength? Which has the greater energy?
950
views
Textbook Question
Which has the higher frequency, infrared light or ultraviolet light? Which has the longer wavelength? Which has the greater energy?
2072
views
1
rank
