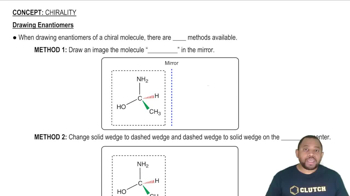
Textbook Question
Propose structures and draw condensed formulas for molecules that meet the following descriptions.
(a) A ketone with the formula C5H10
111
views


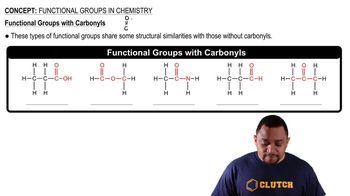
Propose structures and draw condensed formulas for molecules that meet the following descriptions.
(a) A ketone with the formula C5H10
Propose structures and draw condensed formulas for molecules that meet the following descriptions.
(b) An ester with the formula C6H12O2
Propose structures and draw condensed formulas for molecules that meet the following descriptions.
(c) A compound with formula C2H5NO2 that is both an amine and a carboxylic acid
Isooctane, the substance in gasoline from which the term octane rating derives, has the formula C8H18. Each carbon has four covalent bonds, and the atoms are connected in the sequence shown. Draw the complete structural formula of isooctane.