Textbook Question
Identify the group 4A element that best fits each of the following descriptions.
(b) Forms the strongest π bonds
99
views



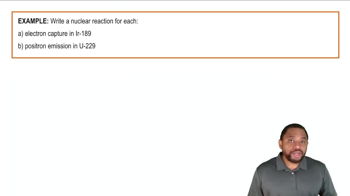
Identify the group 4A element that best fits each of the following descriptions.
(b) Forms the strongest π bonds
Identify the group 4A element that best fits each of the following descriptions.
(c) Is the second most abundant element in the Earth's crust
Select the group 4A element that best fits each of the following descriptions.
(c) Is the second most abundant element in the human body
Identify the group 5A element that best fits each of the following descriptions.
(a) Forms strong π bonds
Could the strain in the P4 molecule be reduced by using sp3 hybrid orbitals in bonding instead of pure p orbitals? Explain.