For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [Pt(NH3)4]2+ (square planar)
 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.114
Problem 21.114
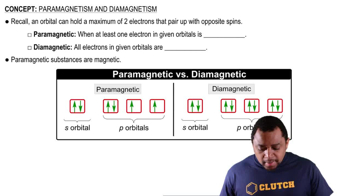


For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(a) [Pt(NH3)4]2+ (square planar)
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(b) [MnCl4]2- (tetrahedral)
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(c) [Co(NCS)4]2- (tetrahedral)
Which of the following complexes are diamagnetic?
(a) [Ni(H2O)6]2+
(b) [Co(CN)6]3-
(c) [HgI4]2- (tetrahedral)
(d) [Cu(NH3)4]2+ (square planar)
Although Cl- is a weak-field ligand and CN- is a strong field ligand, [CrCl6]3- and [Cr(CN)6]3- exhibit approximately the same amount of paramagnetism. Explain.
Draw a crystal field energy-level diagram, and predict the number of unpaired electrons for each of the following:
(a) [Mn(H2O)6]2+