For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(c) [Co(NCS)4]2- (tetrahedral)
 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.116
Problem 21.116
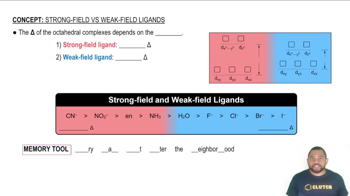
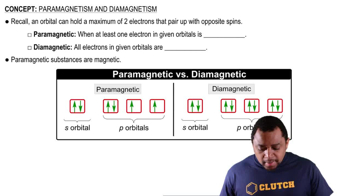
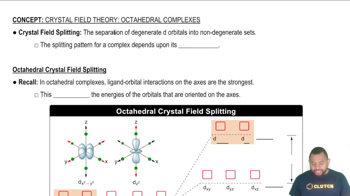
For each of the following complexes, draw a crystal field energy-level diagram, assign the electrons to orbitals, and predict the number of unpaired electrons.
(c) [Co(NCS)4]2- (tetrahedral)
Which of the following complexes are paramagnetic?
(a) [Mn(CN)6]3-
(b) [Zn(NH3)4]2+ (tetrahedral)
(c) [Fe(CN)6]4-
(d) [FeF6]4-
Which of the following complexes are diamagnetic?
(a) [Ni(H2O)6]2+
(b) [Co(CN)6]3-
(c) [HgI4]2- (tetrahedral)
(d) [Cu(NH3)4]2+ (square planar)
Draw a crystal field energy-level diagram, and predict the number of unpaired electrons for each of the following:
(a) [Mn(H2O)6]2+
Explain why [CoCl4]2- (blue) and [Co(H2O)6]2+ (pink) have different colors. Which complex has its absorption bands at longer wavelengths?
Look at the colors of the isomeric complexes in Figure 21.12, and predict which is the stronger field ligand, nitro (-NO2) of nitrito (-ONO). Explain.