Spinach contains a lot of iron but is not a good source of dietary iron because nearly all the iron is tied up in the oxalate complex [Fe(C2O4)3]3-.
(d) Draw the structure of [Fe(C2O4)3]3-. Is the complex chiral or achiral?
 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.138d
Problem 21.138d
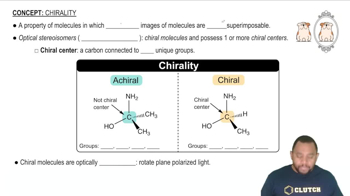


Spinach contains a lot of iron but is not a good source of dietary iron because nearly all the iron is tied up in the oxalate complex [Fe(C2O4)3]3-.
(d) Draw the structure of [Fe(C2O4)3]3-. Is the complex chiral or achiral?
The percent iron in iron ore can be determined by dissolving the ore in acid, then reducing the iron to Fe2+, and finally titrating the Fe2+ with aqueous KMnO4. The reaction products are Fe2+ and Mn2+.
(c) Draw a crystal field energy-level diagram for the reactants and products, MnO4-, 3Fe1H2O2642+, 3Fe1H2O2643+, and 3Mn1H2O2642+, and predict the number of unpaired electrons for each.
The complete reaction of 2.60 g of chromium metal with 50.00 mL of 1.200 M H2SO4 in the absence of air gave a blue solution and a colorless gas that was collected at 25°C and a pressure of 735 mm Hg. (e) When an excess of KCN is added to the solution, the color changes, and the paramagnetism of the solution
decreases. Explain.
In acidic aqueous solution, the complex trans-[Co(en)2Cl1]2+(aq) undergoes the following substitution reaction:
trans-[Co(en)2Cl1]+(aq) + H2O(l) → trans-[Co(en)2(H2O)Cl]2+(aq) + Cl–(aq)
The reaction is first order in trans-[Co(en)2Cl2]+(aq), and the rate constant at 25°C is 3.2×10–5 s–1.
e. Draw a crystal field energy-level diagram for trans-[Co(en)2Cl2]+ that takes account of the fact that Cl– is a weaker-field ligand than ethylenediamine.
Cobalt(III) trifluoroacetylacetonate, Co(tfac)3, is a sixc oordinate, octahedral metal chelate in which three planar, bidentate tfac ligands are attached to a central Co atom:
(a) Draw all possible diastereoisomers and enantiomers of Co(tfac)3.
Cobalt(III) trifluoroacetylacetonate, Co(tfac)3, is a sixcoordinate, octahedral metal chelate in which three planar, bidentate tfac ligands are attached to a central Co atom:
(b) Diastereoisomers A and B have dipole moments of 6.5 D and 3.8 D, respectively. Which of your diastereoisomers is A and which is B?