The percent iron in iron ore can be determined by dissolving the ore in acid, then reducing the iron to Fe2+, and finally titrating the Fe2+ with aqueous KMnO4. The reaction products are Fe2+ and Mn2+.
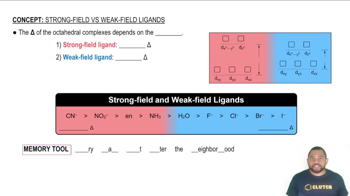
(c) Draw a crystal field energy-level diagram for the reactants and products, MnO4-, 3Fe1H2O2642+, 3Fe1H2O2643+, and 3Mn1H2O2642+, and predict the number of unpaired electrons for each.