Here are the essential concepts you must grasp in order to answer the question correctly.
Polarity
Polarity refers to the distribution of electrical charge over the atoms in a molecule. Polar molecules, like water, have a significant difference in electronegativity between their atoms, leading to partial positive and negative charges. Nonpolar molecules, such as butane, have an even distribution of charge. Understanding polarity is crucial for predicting solubility, as 'like dissolves like' means polar substances dissolve well in polar solvents, while nonpolar substances dissolve better in nonpolar solvents.
Recommended video:
Hydrophobic and Hydrophilic Interactions
Hydrophobic interactions occur between nonpolar molecules that do not interact favorably with water, while hydrophilic interactions occur between polar molecules that can form hydrogen bonds with water. Butane, being a nonpolar hydrocarbon, is hydrophobic and will not dissolve well in water. In contrast, benzene is also nonpolar, making it a more suitable solvent for butane due to similar intermolecular forces.
Recommended video:
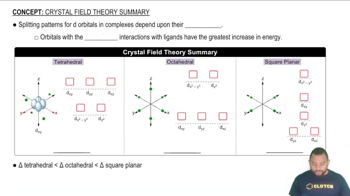
The greatest ligand-orbital interactions result in the greatest increase in energy.
Solubility Principles
Solubility is the ability of a solute to dissolve in a solvent at a given temperature and pressure. Factors influencing solubility include temperature, pressure, and the nature of the solute and solvent. For butane, its solubility is expected to be higher in benzene than in water due to the nonpolar nature of both butane and benzene, which allows for favorable interactions compared to the polar nature of water.
Recommended video:
Uncertainty Principle Formula