Here are the essential concepts you must grasp in order to answer the question correctly.
Polarity and Solubility
The principle of 'like dissolves like' states that polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Water is a polar solvent, meaning it effectively dissolves ionic and polar compounds, but not nonpolar substances. Understanding the polarity of the compounds in question is essential for predicting their solubility in water.
Recommended video:
Ionic Compounds and Solubility
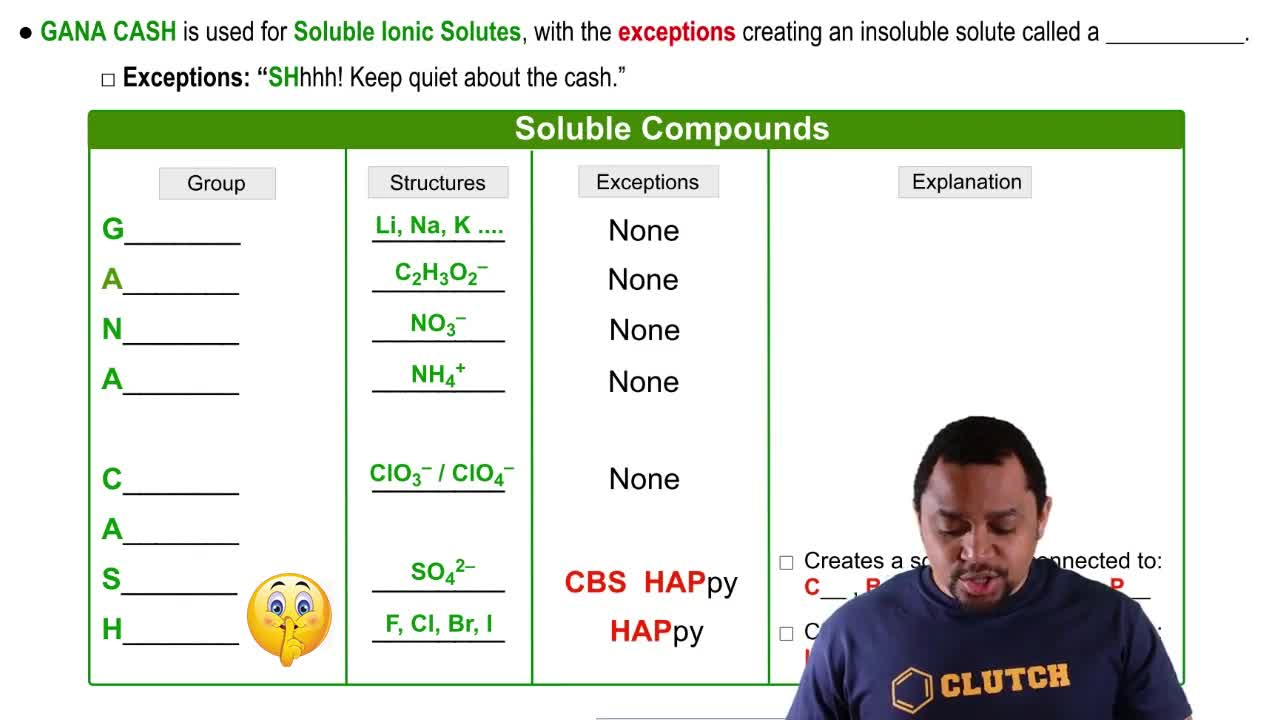
Ionic compounds, such as KBr, typically have high solubility in water due to the strong interactions between the ions and the polar water molecules. When dissolved, the ionic bonds break, allowing the ions to disperse in the solution. This contrasts with covalent compounds, which may not dissociate in water.
Recommended video:
Nonpolar Compounds and Solubility
Nonpolar compounds, like toluene (C7H8), have little to no interaction with polar solvents like water, leading to low solubility. Toluene is a hydrocarbon and does not form hydrogen bonds with water, making it less soluble compared to ionic compounds. Recognizing the nonpolar nature of toluene is crucial for understanding its position in the solubility order.
Recommended video: