Here are the essential concepts you must grasp in order to answer the question correctly.
Density Conversion
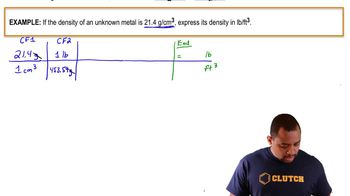
Density is defined as mass per unit volume, typically expressed in units like grams per cubic centimeter (g/cm³) or grams per cubic meter (g/m³). To convert between these units, one must recognize that 1 g/cm³ is equivalent to 1000 g/m³. Therefore, understanding how to convert density from mg/cm³ to g/m³ is essential for solving the problem.
Recommended video:
Density Conversion Example
Unit Prefixes
Unit prefixes such as 'milli-' (m) and 'kilo-' (k) are used to denote specific powers of ten. For example, 'milli-' indicates a factor of 10^-3, meaning 1 mg is 0.001 g. Recognizing these prefixes helps in accurately converting measurements and understanding the scale of the values involved in the problem.
Recommended video:
Aerogel Properties
Aerogels are unique materials characterized by their low density and high porosity, making them excellent insulators. Their structure, which consists mostly of air, contributes to their thermal insulating properties. Understanding the physical properties of aerogels can provide context for their applications and the significance of their density in practical scenarios.
Recommended video: