Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Reactions
Nuclear reactions involve changes in an atom's nucleus, resulting in the transformation of one element into another. In positron emission and electron capture, the nucleus undergoes a process that alters its composition, specifically the number of protons and neutrons, leading to the formation of a different element.
Recommended video:
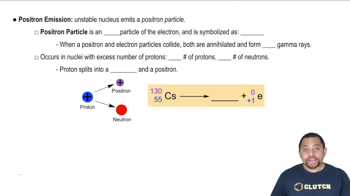
Positron Emission
Positron emission is a type of beta decay where a proton in the nucleus is converted into a neutron, releasing a positron (the antimatter counterpart of an electron) and a neutrino. This process decreases the atomic number by one, resulting in a new element that is one position to the left on the periodic table.
Recommended video:
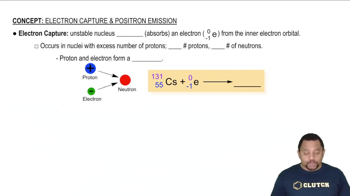
Electron Capture
Electron capture occurs when an inner orbital electron is captured by the nucleus, where it combines with a proton to form a neutron and a neutrino. This reaction also reduces the atomic number by one, leading to the formation of a new element, similar to positron emission, but through a different mechanism.
Recommended video:
 Verified step by step guidance
Verified step by step guidance