Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Reactions
Nuclear reactions involve changes in an atom's nucleus and can result in the transformation of one element into another. These reactions include processes such as alpha decay, beta decay, and positron emission, where particles are emitted from the nucleus, altering the atomic number and mass number of the original atom.
Recommended video:
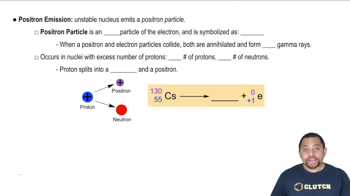
Positron Emission
Positron emission is a type of beta decay where a proton in the nucleus is converted into a neutron, releasing a positron (the antimatter counterpart of an electron) and a neutrino. This process decreases the atomic number by one while keeping the mass number unchanged, resulting in the formation of a new element.
Recommended video:
Balancing Nuclear Equations
Balancing nuclear equations requires ensuring that the total number of protons and neutrons is the same on both sides of the equation. This involves accounting for the emitted particles and the resulting nuclide, maintaining conservation of mass and charge throughout the reaction.
Recommended video:
Balancing Chemical Equations