Here are the essential concepts you must grasp in order to answer the question correctly.
Hall–Héroult Process
The Hall–Héroult process is an industrial method for extracting aluminum from its ore, bauxite. It involves the electrolysis of aluminum oxide dissolved in molten cryolite, where aluminum ions are reduced to form aluminum metal at the cathode. Understanding this process is crucial for calculating the current required, as it directly relates to the amount of aluminum produced and the efficiency of the electrolysis.
Recommended video:
Faraday's Laws of Electrolysis
Faraday's Laws of Electrolysis describe the relationship between the amount of substance produced at an electrode during electrolysis and the quantity of electric charge passed through the electrolyte. The first law states that the mass of a substance altered at an electrode is proportional to the total electric charge passed. This principle is essential for determining the current needed to produce a specific mass of aluminum in the Hall–Héroult process.
Recommended video:
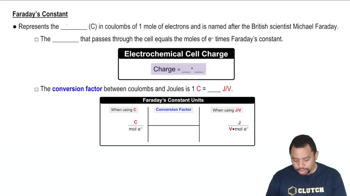
Faraday's Constant in Electrochemistry
Current Calculation
To calculate the current required for electrolysis, one must consider the molar mass of aluminum and the charge needed to reduce aluminum ions. The formula I = (n * F) / t relates current (I) to the number of moles (n), Faraday's constant (F), and time (t). This calculation is vital for determining the amperage necessary to achieve the desired production rate of aluminum, ensuring efficient operation of the electrolysis process.
Recommended video:
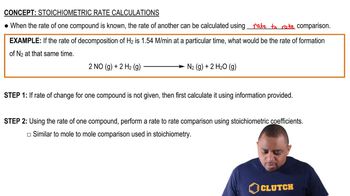
Stoichiometric Rate Calculations
 Verified step by step guidance
Verified step by step guidance