Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolysis
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction. In the case of molten NaCl, electrolysis separates sodium ions (Na+) and chloride ions (Cl-) at the anode and cathode, respectively. This process is essential for producing sodium metal and chlorine gas from sodium chloride.
Recommended video:
Faraday's Laws of Electrolysis
Faraday's Laws of Electrolysis quantify the relationship between the amount of substance produced at an electrode and the electric charge passed through the electrolyte. The first law states that the mass of a substance produced is directly proportional to the electric charge. This principle is crucial for calculating the time required for electrolysis based on current and the amount of sodium desired.
Recommended video:
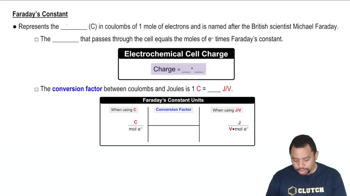
Faraday's Constant in Electrochemistry
Stoichiometry of Gases
Stoichiometry of gases involves the relationships between the volumes of gases and the amounts of substances in a chemical reaction, particularly under standard temperature and pressure (STP). At STP, one mole of any gas occupies 22.4 liters. This concept is vital for determining the volume of chlorine gas produced as a by-product during the electrolysis of NaCl.
Recommended video:
 Verified step by step guidance
Verified step by step guidance