Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolysis
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction. In this process, an electric current is passed through an electrolyte solution, causing the ions to migrate towards the electrodes, where they undergo reduction or oxidation. The amount of substance produced at the electrodes can be calculated using Faraday's laws of electrolysis.
Recommended video:
Faraday's Laws of Electrolysis
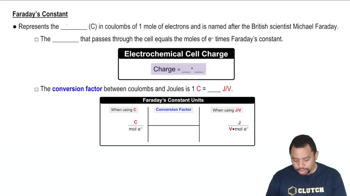
Faraday's laws of electrolysis quantify the relationship between the amount of substance transformed at an electrode and the electric charge passed through the electrolyte. The first law states that the mass of a substance deposited or dissolved at an electrode is directly proportional to the quantity of electricity that passes through the electrolyte. The second law states that the mass of different substances deposited by the same quantity of electricity is proportional to their equivalent weights.
Recommended video:
Faraday's Constant in Electrochemistry
Molar Mass and Stoichiometry
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole. In electrolysis, stoichiometry is used to relate the amount of metal produced to the charge passed through the solution. By knowing the mass of the metal obtained and the current used, one can calculate the molar mass of the metal ion, which helps identify the metal ion M2+ in the reaction.
Recommended video:
 Verified step by step guidance
Verified step by step guidance